Bracco Diagnostics' MultiHance Contrast Agent Earns Expanded Labeling for Pediatric MRI
Bracco Diagnostics Inc. announced the labeling of its contrast agent MultiHance has obtained U.S. Food and Drug Administration (FDA) approval for an extension to include magnetic resonance imaging (MRI) of the central nervous system (CNS) in pediatric patients younger than 2 years of age (including term neonates). The agent may now be used in this patient population to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine and associated tissues.

Bracco Diagnostics' MultiHance Contrast Agent Earns Expanded Labeling for Pediatric MRI
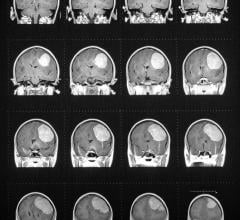
Bracco's MultiHance to Show Improved MR Visualization

Magnetic Resonance Angiography of the Thoracic Vasculature: Technique and Applications - Ludwig - 2020 - Journal of Magnetic Resonance Imaging - Wiley Online Library

Imaging Technology News - Magnetic Resonance Imaging (MRI)

Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging
Bracco Diagnostics Imaging Technology News

Pediatric magnetic resonance angiography: to contrast or not to contrast

Pediatric magnetic resonance angiography: to contrast or not to contrast

A liposomal Gd contrast agent does not cross the mouse placental barrier