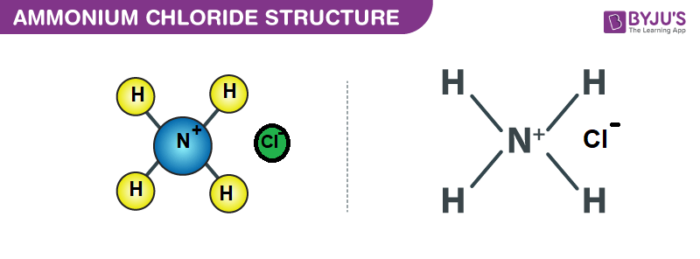
Ammonium Chloride (NH4Cl) - Structure, Properties, Preparation, Uses, Health Risk & FAQs of Ammonium Chloride.
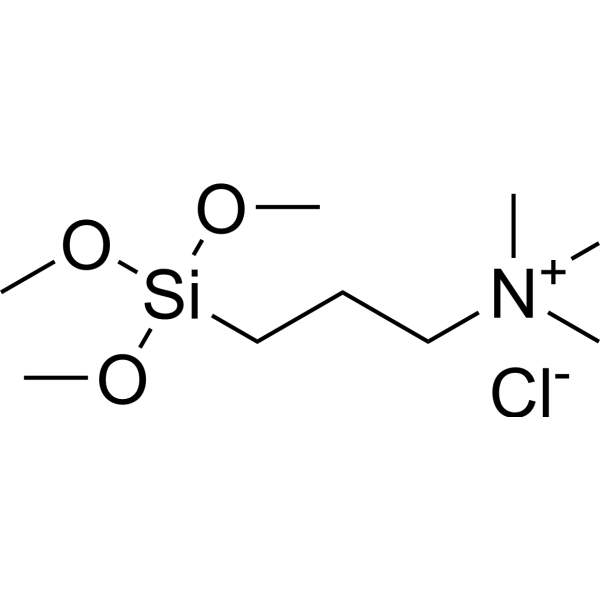
Ammonium Chloride (NH4Cl) - Ammonium chloride is an inorganic compound with formula NH4Cl. In its pure form, it is white crystalline salt. Ammonium chloride is used in veterinary medicine in the prevention of urinary stones in sheep, goats, and cattle. To learn more about the Structure, Properties, Preparation, Uses and FAQs of Ammonium Chloride (NH4Cl), Visit BYJU’S for more content.
Ammonium Chloride (NH4Cl) - Ammonium chloride is an inorganic compound with formula NH4Cl. In its pure form, it is white crystalline salt. Ammonium chloride is used in veterinary medicine in the prevention of urinary stones in sheep, goats, and cattle. To learn more about the Structure, Properties, Preparation, Uses and FAQs of Ammonium Chloride (NH4Cl), Visit BYJU’S for more content.
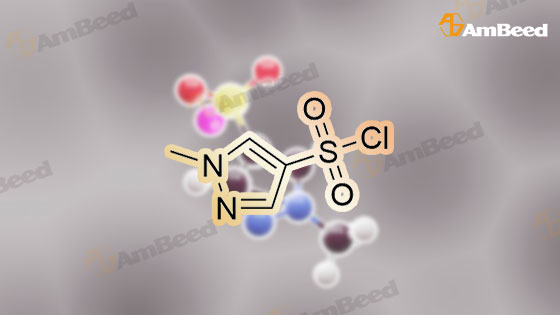
288148-34-5, 1-Methyl-1H-pyrazole-4-sulfonyl chloride

PDF) Review of Ammonium Chloride–Water Solution Properties

High-Pressure Synthesis and Crystal Structure of MoC-Type Tungsten Nitride by Nitridation with Ammonium Chloride
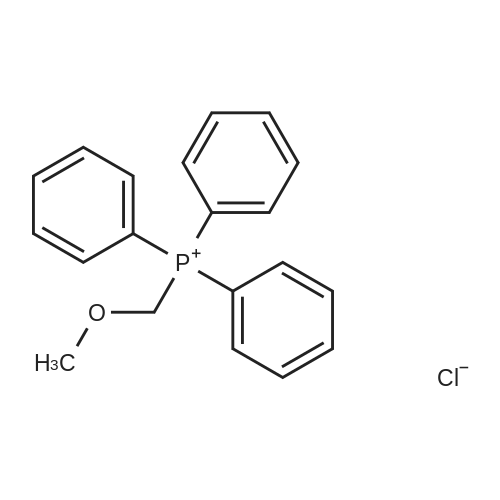
4009-98-7, (Methoxymethyl)triphenylphosphonium chloride
Ammonium Chloride, NH4Cl

Ammonium chloride, Formula, Uses, & Facts

Use of NH4Cl for activation of carbon xerogel to prepare a novel efficacious adsorbent for benzene removal from contaminated air streams in a fixed-bed column

Ammonium Chloride Formula - Structure, Properties, Uses, Sample Questions - GeeksforGeeks

Ammonium chloride NH₄Cl : Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure –