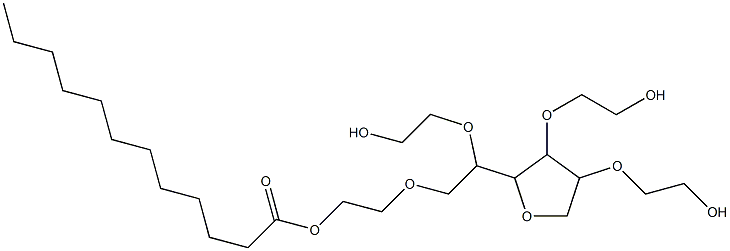
Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism

Improving Prediction of Free Fatty Acid Particle Formation in Biopharmaceutical Drug Products: Incorporating Ester Distribution during Polysorbate 20 Degradation

Hydrolytic polysorbate 20 degradation – Sensitive detection of free fatty acids in biopharmaceuticals via UPLC-QDa analytics with isolator column - ScienceDirect

Hydrolytic polysorbate 20 degradation – Sensitive detection of free fatty acids in biopharmaceuticals via UPLC-QDa analytics with isolator column - ScienceDirect
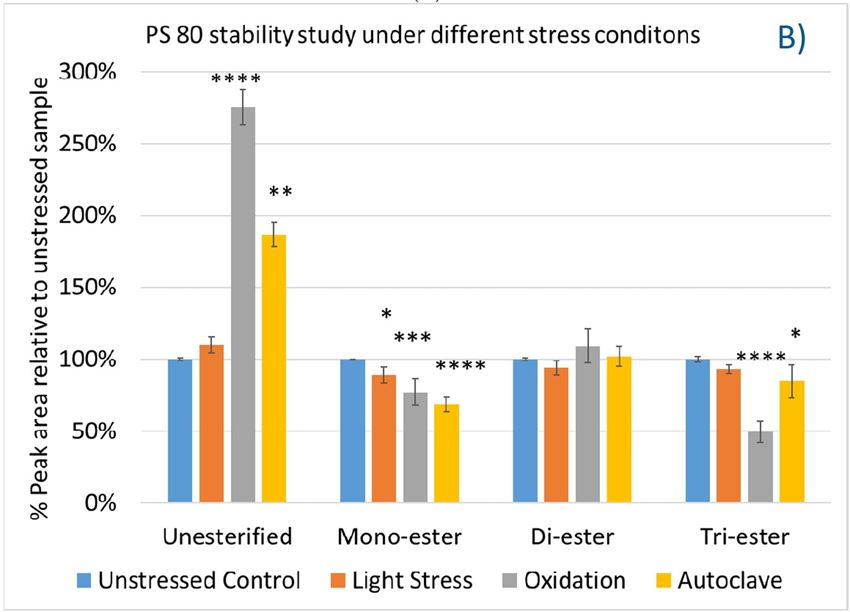
Polysorbates versus Hydroxypropyl Beta-Cyclodextrin: Comparative Study on Excipient Stability and Stabilization Benefits on Monoclonal Antibodies
Considerations for the Use of Polysorbates in Biopharmaceuticals

Novel markers to track oxidative polysorbate degradation in pharmaceutical formulations - ScienceDirect

Full article: Prediction of long-term polysorbate degradation according to short-term degradation kinetics

Characterization of Polysorbate Ester Fractions and Implications

Polysorbate Degradation and Particle Formation in a High Concentration mAb: Formulation Strategies to Minimize Effect of Enzymatic Polysorbate Degradation - ScienceDirect