Clinical outcomes subject to formal monitoring in the WHI Hormone
Download Table | Clinical outcomes subject to formal monitoring in the WHI Hormone Therapy Trial component from publication: Monitoring and reporting of the Women's Health Initiative randomized hormone therapy trials | The Women's Health Initiative (WHI) randomized trial of estrogen plus progestin (E + P) was terminated early based on an assessment of harms exceeding benefits for disease prevention. The results contravened prevailing wisdom and a large body of literature regarding benefits | Women's Health, Estrogen Replacement Therapy and Estrogens | ResearchGate, the professional network for scientists.

Association of glycaemic index and glycaemic load with type 2

Oral Presentations 2022 AANS Annual Scientific Meeting in: Journal
Full article: Safety and benefit considerations for menopausal

Using Implementation Science Frameworks to Guide the Use of
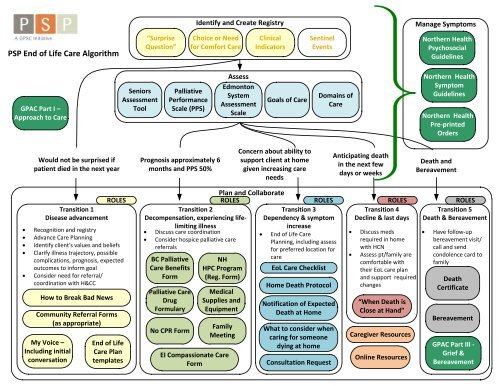
psp eol algorithm - northern health - GPSC
Clinical Trial An Assessment of the NIH Women's Health

Health Equity - Kaiser Permanente Division of Research

Impact of Lifetime Obesity on Urinary Incontinence in the Women's

IJMS, Free Full-Text

Establishing the risk related to hormone replacement therapy and