What you need to know about the PRISMA reporting guidelines

PDF) The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration

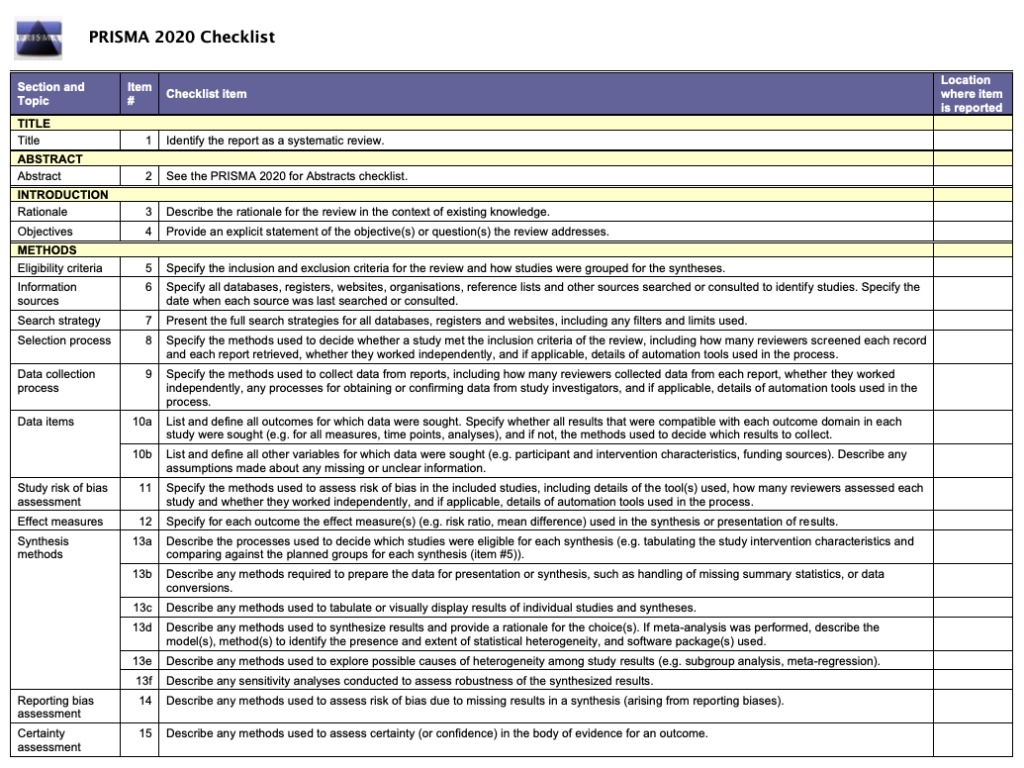
PRISMA 2020: updated guidelines for reporting systematic reviews and meta-analyses
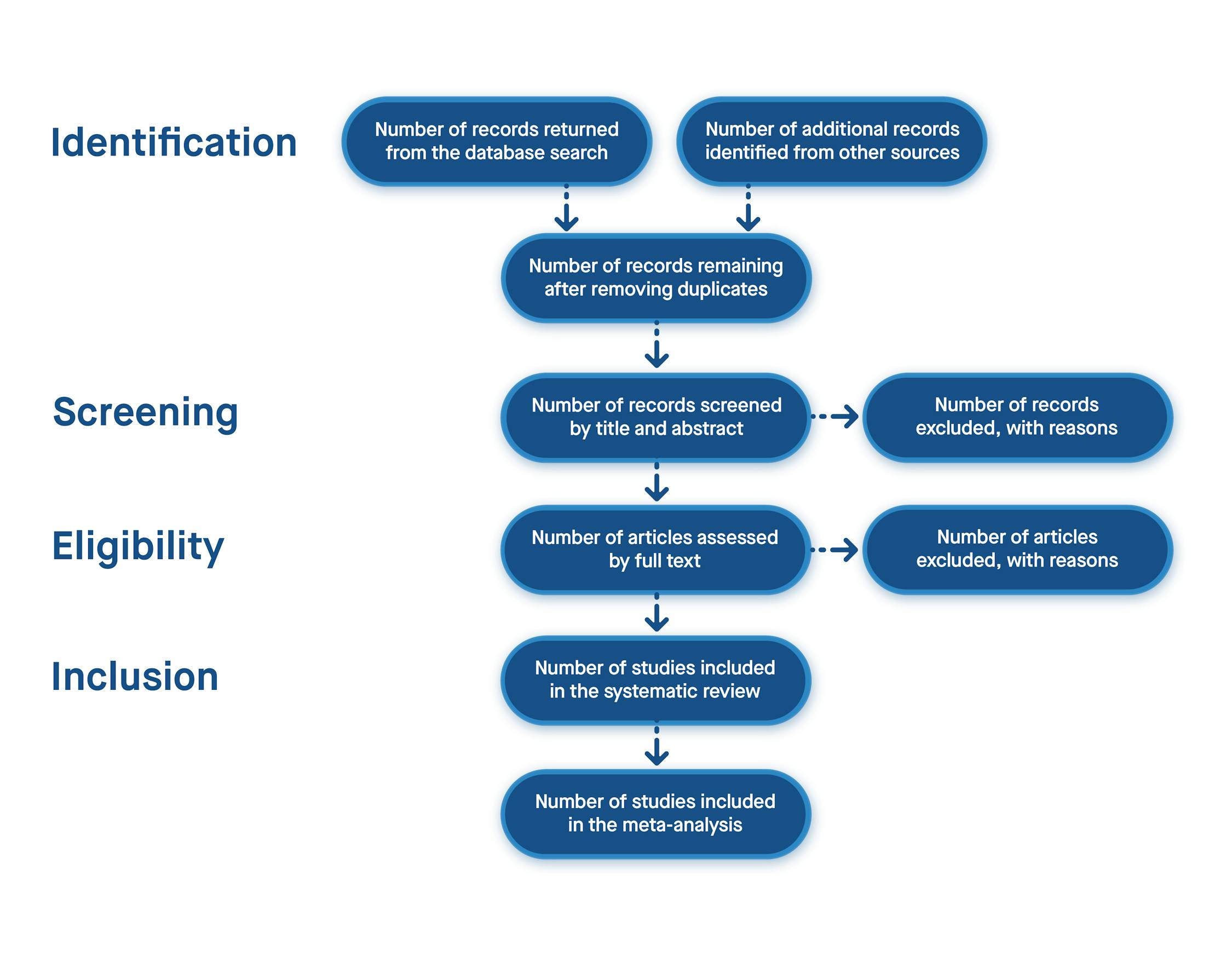
How to Create an Effective PRISMA Flow Diagram

The PRISMA 2020 statement: An improved reporting guideline for systematic reviews

PDF) PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews
Preferred Reporting Items for Systematic Reviews and Meta-Analyses

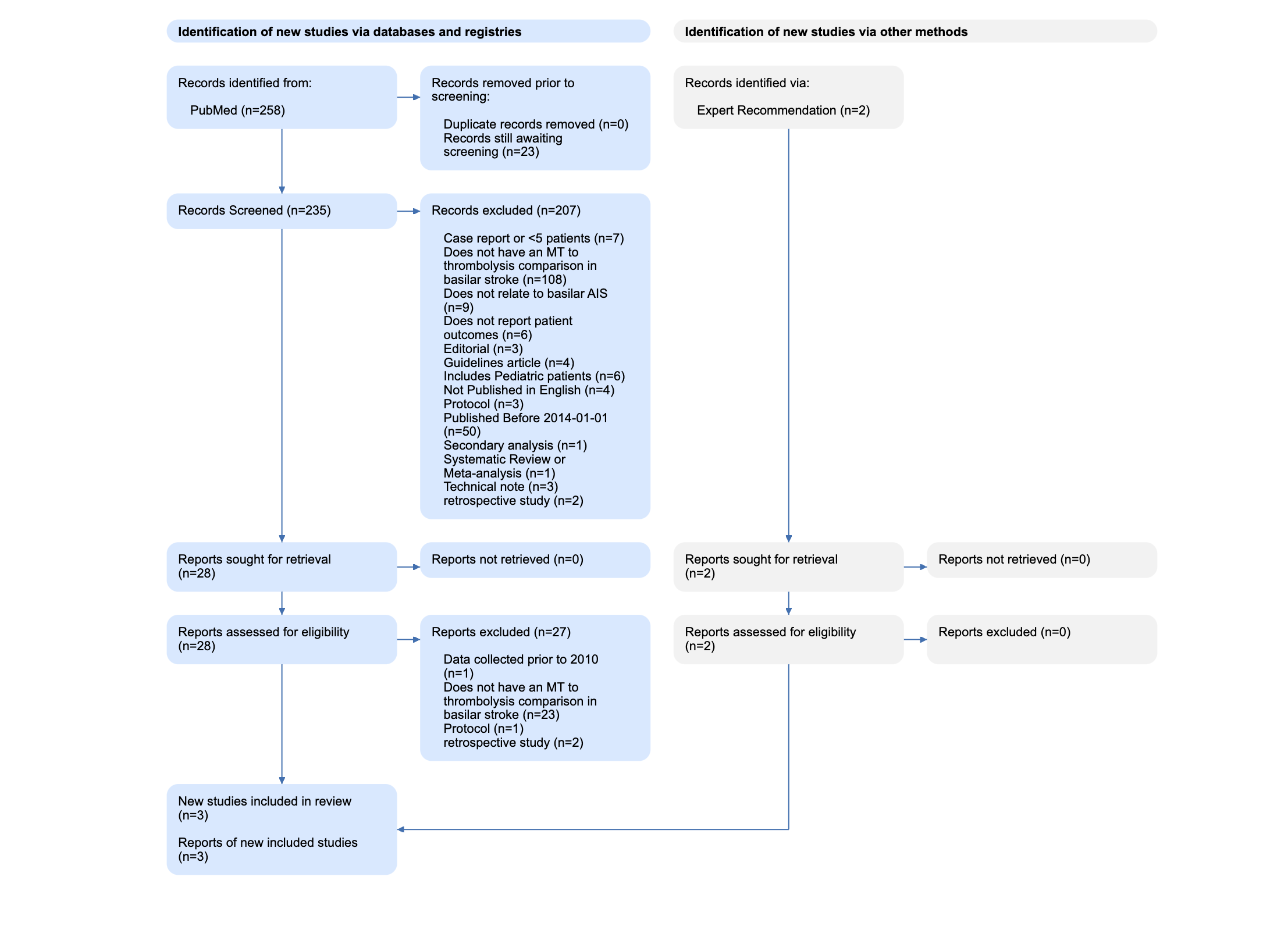
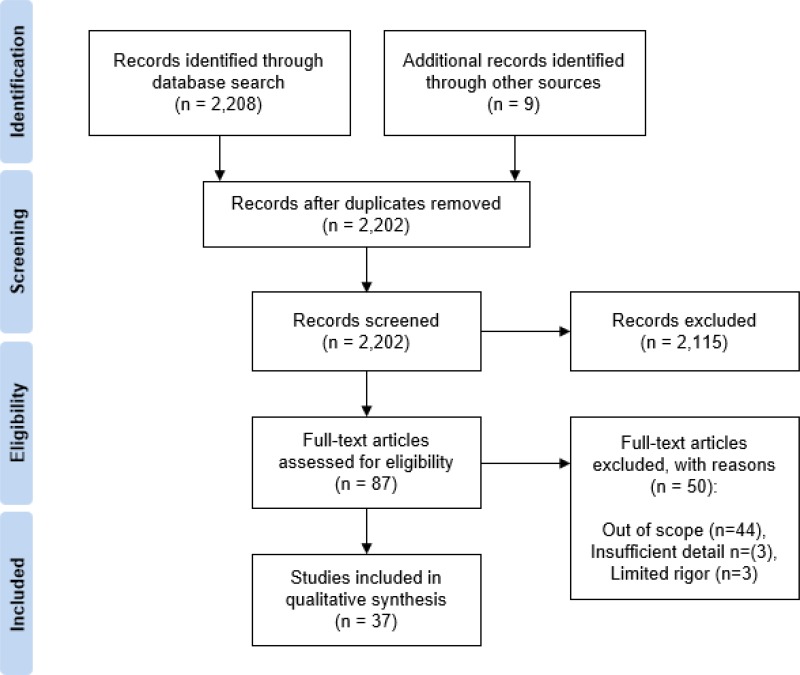
Flowchart of study selection according to PrisMa guidelines.

Adherence to the PRISMA-P 2015 reporting guideline was inadequate in systematic review protocols - ScienceDirect

PDF] PRISMA-Equity 2012 Extension: Reporting Guidelines for Systematic Reviews with a Focus on Health Equity
PRISMA Flow Diagrams - Making Healthcare Safer III: A Critical Analysis of Existing and Emerging Patient Safety Practices - NCBI Bookshelf

PRISMA flow chart showing study screening process. PRISMA indicates

What you need to know about the PRISMA reporting guidelines - Covidence

Study protocol for developing, piloting and disseminating the PRISMA-COSMIN guideline: a new reporting guideline for systematic reviews of outcome measurement instruments, Systematic Reviews